WHO panel is reviewing whether COVID vaccines need to be updated
[ad_1]
In the latest sign that the coronavirus pandemic is not yet over, the World Health Organization said this week that a panel of experts is reviewing whether COVID vaccines need to be updated.
The WHO panel, which is called the Technical Advisory Group on COVID-19 Vaccine Composition, outlined the process in commentary in the journal Nature Medicine, in which they agreed the vaccines are still offering a high level of protection against severe disease caused by all of the variants, including omicron, which is dominant globally.
“However, there has been continuous and substantial evolution of SARS-CoV-2 since the virus emerged, posing challenges to the ongoing public health response, including ensuring that vaccines continue to provide protection,” the authors wrote.
As the virus continues to spread by community transmission, further evolution is possible and the trajectory and timeline of virus evolution is uncertain, they added.
“The current approach to vaccine antigen composition may not be sustainable in the long term, given the length of time for vaccine development, the paucity of surveillance data globally and the regulatory requirements in different countries,” they wrote.
The WHO is now monitoring seven omicron subvariants, according to its weekly epidemiological update, up from four a week ago. The seven are BF.7, BQ.1, BA.2.75, CH.1.1, XBB, XBB.1.5 and XBF.
“These variants are included due to their observed transmission advantage relative to other circulating variants and additional amino acid changes that are known or suspected to confer fitness advantage,” said the update.
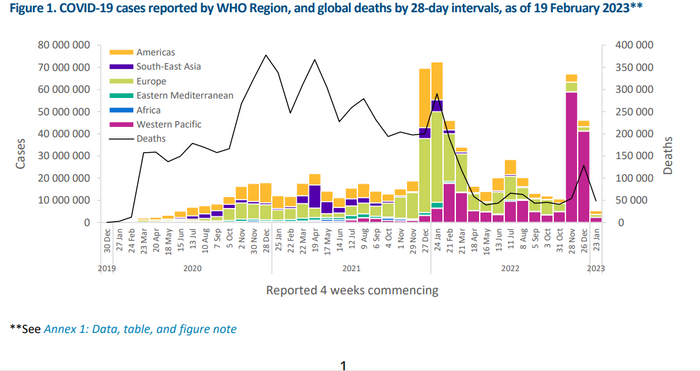
The agency said almost 5.3 million new COVID cases were reported in the 28-day period through Feb. 19, down 89% from the previous 28-day period. Almost 48,000 deaths were reported in the same time frame, down 62% from the previous 28 days.
As usual, it cautioned that the numbers may be distorted by a pullback in testing and surveillance and noted that the numbers are higher in prevalance surveys.
Source: WHO
Meanwhile, the seven-day average of new U.S. cases of COVID stood at 34,679 on Thursday, according to a New York Times tracker. That’s down 13% from two weeks ago.
The number of people hospitalized for COVID fell 5% to 27,966, while the number of fatalities fell 28% to 330.
Cases are currently rising in 17 states and are flat in Oregon and South Dakota. Georgia leads with a 75% increase from two weeks ago. On a per capita basis, Delaware leads with 18 cases per 100,000 residents, the tracker shows.
Other COVID-19 news you should know about:
• Moderna’s
MRNA,
stock took a tumble on Thursday when the vaccine maker posted fourth-quarter earnings that fell short of estimates, amid a steep decline in sales of its COVID vaccine, Spikevax — which is the company’s sole product to have won regulatory approval. Investors were further disappointed by the Cambridge, Mass.-based company’s guidance for about $5 billion in COVID sales in 2023, which is almost $3 billion below the FactSet consensus of $7.9 billion. The guidance is backloaded to the latter half of the year, with product sales in the first half expected to come to about $2 billion, also below the $3.2 billion forecast by FactSet analysts. Investors weighing in on the numbers said it seems the COVID boost is fading for Moderna, hardly a surprise as the pandemic shifts to a new phase and President Joe Biden prepares to end the twin emergencies that gave the government special powers to manage it.
• Gilead Sciences Inc.
GILD,
said this week that its COVID-19 drug can help reduce deaths and readmission rates among patients hospitalized with COVID-19 who received the drug, remdesivir, shortly after their hospital admission. That was regardless of disease severity and variant, said Gilead, which markets the drug as Veklury and based its findings on three studies. The treatment also led to fewer deaths among immunocompromised patients, such as people with cancer or HIV, the company said. Gilead earlier this month reported another drop in Veklury sales.
• Merck & Co.
MRK,
and privately held Ridgeback Biotherapeutics said a panel advising the European regulator has recommended refusing marketing authorization for their jointly developed COVID antiviral. The companies said they would appeal the decision and request a review of the opinion, which was offered by the Committee for Medicinal Products for Human Use of the European Medicines Agency. The therapy, called Lagevrio or molnupiravir, has been approved or authorized for use in more than 25 countries, including the U.S., Australia, Japan, the U.K. and China. It is mostly used in adults with mild to moderate symptoms who are at risk of developing severe disease.
• Nearly 30 million Americans who got extra government help with grocery bills during the pandemic will soon see that additional aid end — and there’s a big push to make sure they’re not surprised by the change, the Associated Press reported. Officials in 32 states and other jurisdictions have been using texts, voicemails, snail mail, flyers and social-media posts — all in multiple languages — to let recipients know that their extra food stamps will end after February’s payments. “One of the scenarios you don’t want to see is the first time they’re aware of it is in the checkout line at the grocery store,” said Ellen Vollinger, an official with the nonprofit Food Research & Action Center.
Here’s what the numbers say:
The global tally of confirmed COVID-19 cases topped 674.8 million on Friday, while the death toll rose above 6.86 million, according to data aggregated by Johns Hopkins University.
The U.S. leads the world with 103.4 million cases and 1,119,508 fatalities.
The CDC’s tracker shows that 229.9 million people living in the U.S., equal to 69.3% of the total population, are fully vaccinated, meaning they have had their primary shots.
So far, just 53.4 million Americans, equal to 16.1% of the overall population, have had the updated COVID booster that targets both the original virus and the omicron variants.
[ad_2]
Source link



